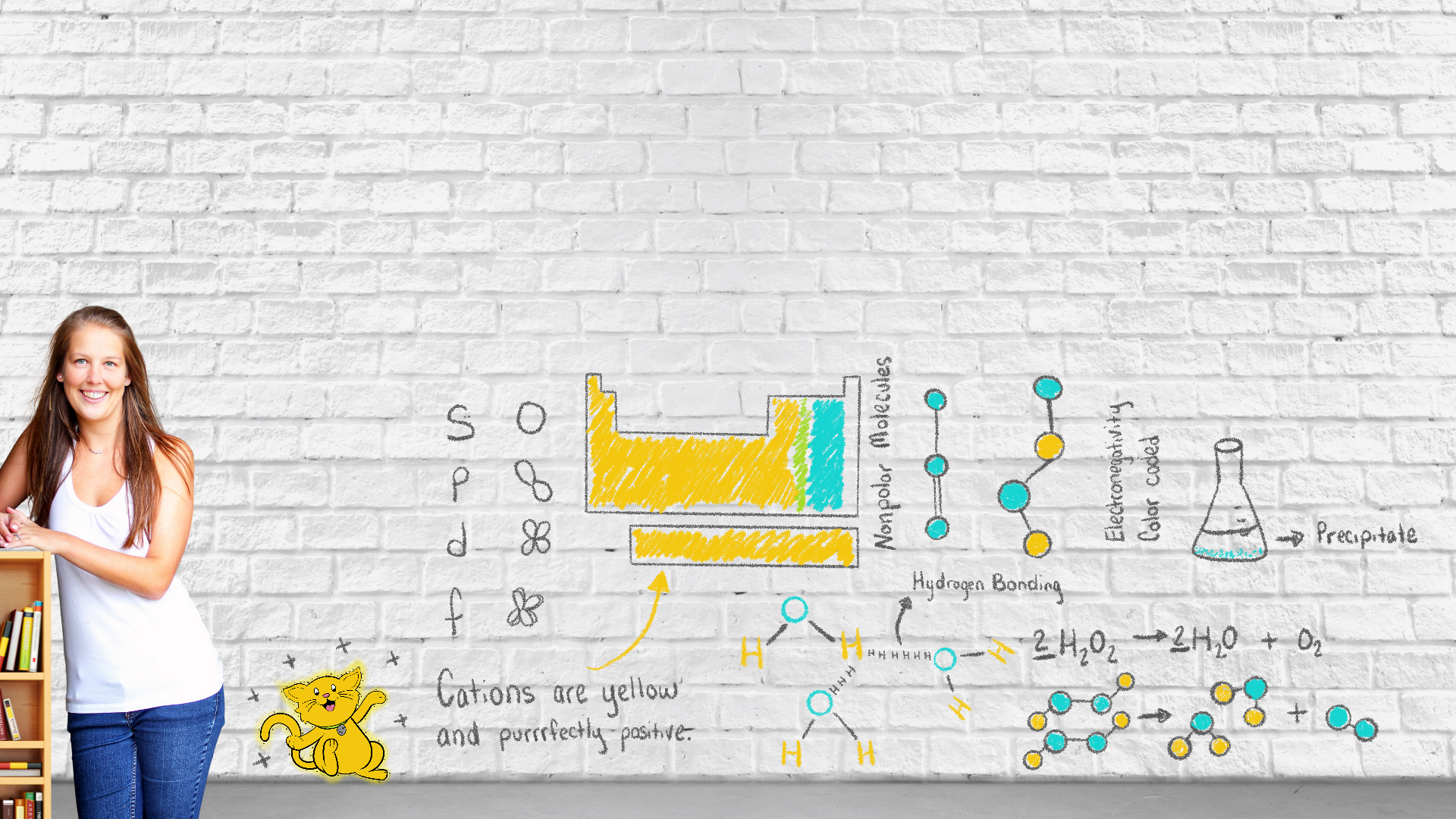
What if you could teach high school chemistry curriculum better through illustrations?
You always wanted your chemistry classroom to be like Mrs. Frizzle’s, but between state standards, lack of planning time, committees, and eating lunch at your desk, you feel more like the teacher on Charlie Brown.
Let's change that.
You know that topic? The one you rush through hoping high school chemistry students won’t notice how much you hate it too? It doesn’t have to be misery. With a few tweaks and teaching ideas, you could master it in a way that lights up students in your classroom.
Learn More About Teaching Difficult Chemistry Topics
Teaching chemistry is hard. Nevermind your students paying attention when provided chemistry curriculum materials are boring and complicated. Which is why these worksheets are fun and engaging. There are traditional style chemistry worksheets in the store. But there are also creative worksheets like doodle notes and color coding worksheets. There are also worksheets that illustrate step by step processes that your school’s McBoring Hill textbook just can’t match.
Shop High School Chemistry Curriculum

Sample chemistry curriculum for free to see which product line fits your teaching style.
You should only use worksheets that enhance your style.
“Thank you for your emails. I just love them and the ideas that pour from them.”
—Angela B.








